Current options for vascular replacements have significant limitations for patients, who often face a cycle of complications and reinterventions.
Xeltis aims to break this cycle by providing new, transformational treatment options.
Xeltis’ current focus is on developing medical devices for three areas of high unmet need.
Vascular access for end-stage renal disease patients requiring hemodialysis
Coronary arterial bypass surgery for cardiovascular disease patients
Pulmonary heart valve for patients with congenital heart malformations requiring valve replacement surgery
The Challenge
Hemodialysis access in end-stage renal disease patients
End-stage renal disease is a devastating condition impacting 850 million patients.
It is projected to be the fifth leading cause of death globally by 2040, with less than 25% of patients surviving five years.
Associated healthcare costs are significant, with $140 billion spent on chronic kidney disease globally each year, with $50 billion on dialysis alone.
To manage the disease, 3.5 million patients each year rely on routine dialysis which requires a way to access the blood for regular hemodialysis. This patient population is growing by 6% annually.
The current options for patients requiring hemodialysis access each year are a temporary catheter, AV fistula or an AV graft. Each is associated with significant limitations.

CURRENT OPTIONS
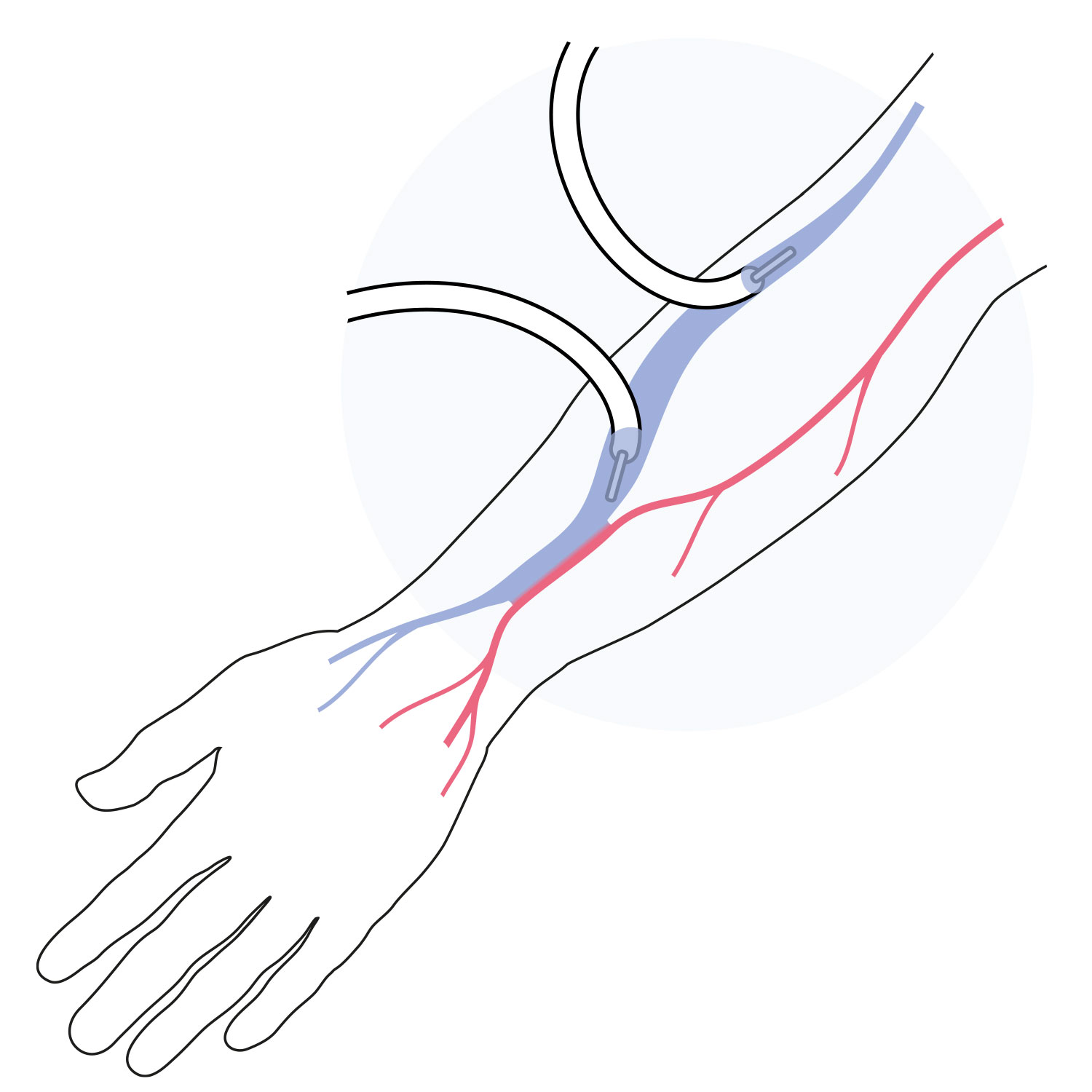
AVF
An arteriovenous fistula (AVF) is a passage created by joining and enlarging an artery and a vein in the patient’s arm.
It is generally a longer lasting option.
Disadvantages
- Takes time to develop or mature:
4 weeks to 4 months - Many fail to mature (up to 60%)
AVG
An arteriovenous graft (AVG) is a small plastic tube implanted in the patient’s arm to connect an artery and a vein.
Most grafts can be used between a few days and within three weeks after implanting.
Disadvantages
- Risk of occlusion and early abandonment
- Increases risk of infection and blood clot
CVC
A central venous catheter (CVC) is a larger tube implanted in the patient’s neck to provide access to larger veins.
It provides immediate dialysis access.
Disadvantages
- Has more than 20% risk of infection, as its tip is located near the heart
6-month FIH data
- Excellent patency and safety profile
- Low reintervention rates
- 0% infections
*presented at VAS in Porto 2023 by Prof. Matteo Tozzi
The Solution
Vascular Access Conduit
aXess is a restorative conduit which enables the creation of a new, long-term living vessel for hemodialysis vascular access.
It combines the safety and patency of a fistula with the speed to treatment of an AV graft. The aXess vascular access conduit offers an improved dialysis patient experience and avoids the frequent reinterventions and complications, such as infections, faced by renal disease patients.
A first-in-human trial of aXess demonstrated a significant improvement in performance compared to hemodialysis vascular access solutions.
A pivotal trial of aXess is currently enrolling in up to 110 patients in nine EU countries.
Go to axesspivotal.com for more details.
The Challenge
Coronary artery bypass surgery
Cardiovascular disease is the leading cause of mortality globally, linked to 17.9 million deaths each year, growing at a rate of 9% annually.
There are 1 million CABG patients each year
20
of vein grafts fail within a year
75
of vein grafts fail within 10 years
40
suffer complications
Cardiovascular diseases have a huge impact on the global healthcare system, with the costs of cardiovascular disease related healthcare totalling €111 billion a year in Europe and $318 billion in the United States.
In the case of multivessel occlusions around the heart, a coronary artery bypass graft (CABG) is needed to divert blood around blockages in order to improve blood flow and the supply of oxygen to the heart.
Coronary artery bypass surgery is the most common cardiac surgery performed worldwide, with around 1 million patients each year.
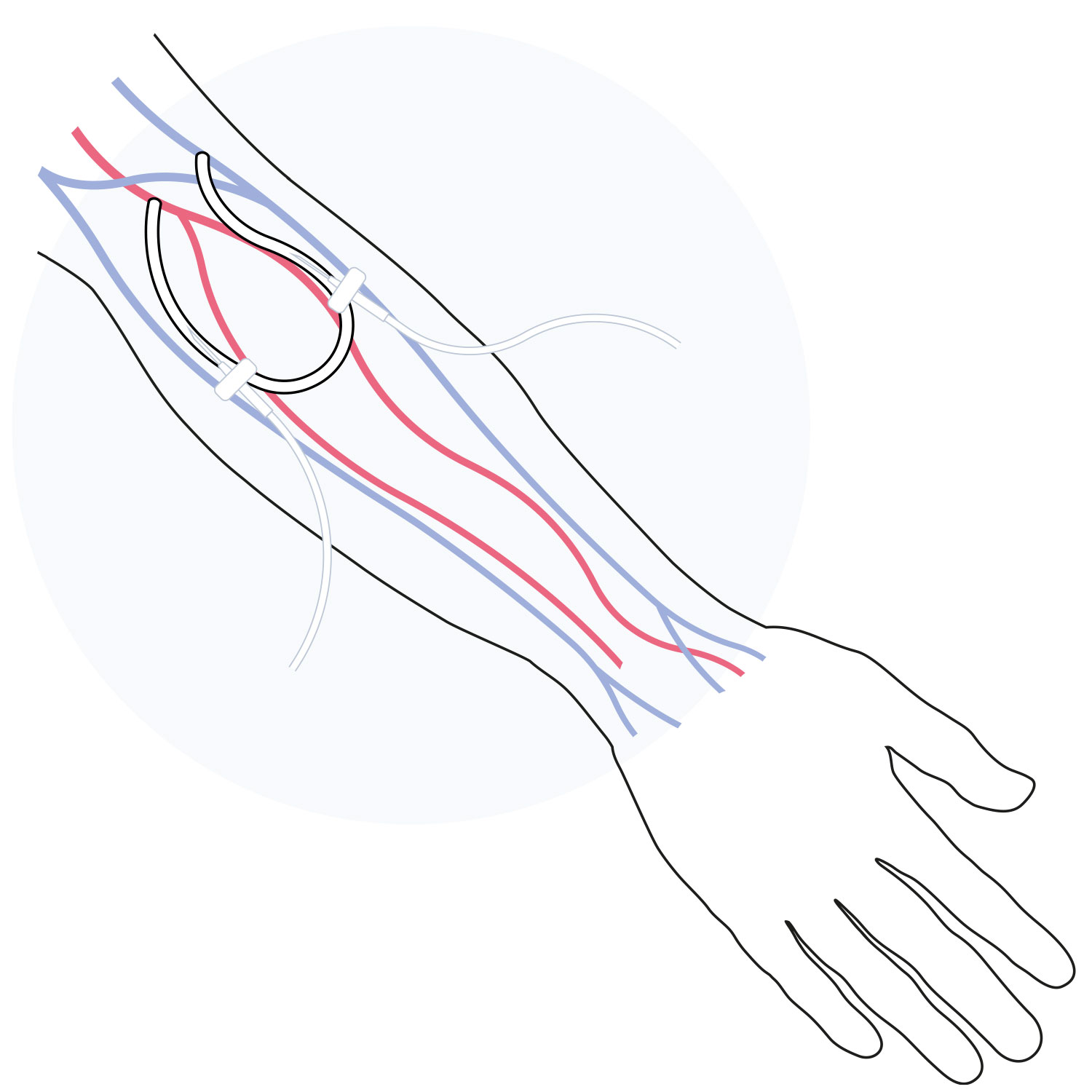
The CABG procedure means that in 80% of the cases implanting a patient’s own vein is the only solution, typically from the leg, to divert blood around blocked arteries. This is an invasive process, often requiring multiple painful vein grafts (on average 2 to 4), with a high risk of complications – 20% of such grafts fail within one year.
The Solution
Coronary Artery Bypass Conduit
Xabg is a restorative conduit which enables continued blood flow in coronary artery disease patients.
Over time, a new long-term living vessel is created. Xeltis’ solution has the potential to transform the bypass surgery landscape, eliminating the need for vein harvesting and its associated burden on patients and healthcare systems.
Proof-of-concept and preclinical trials have been completed in animals, demonstrating the conduit has remained open for 6-12 months, with healthy tissue regeneration in and around the vessel leading to long-term durability.
A first-in-human trial is underway. Click here to find out more.
The Challenge
Pulmonary heart valve replacement
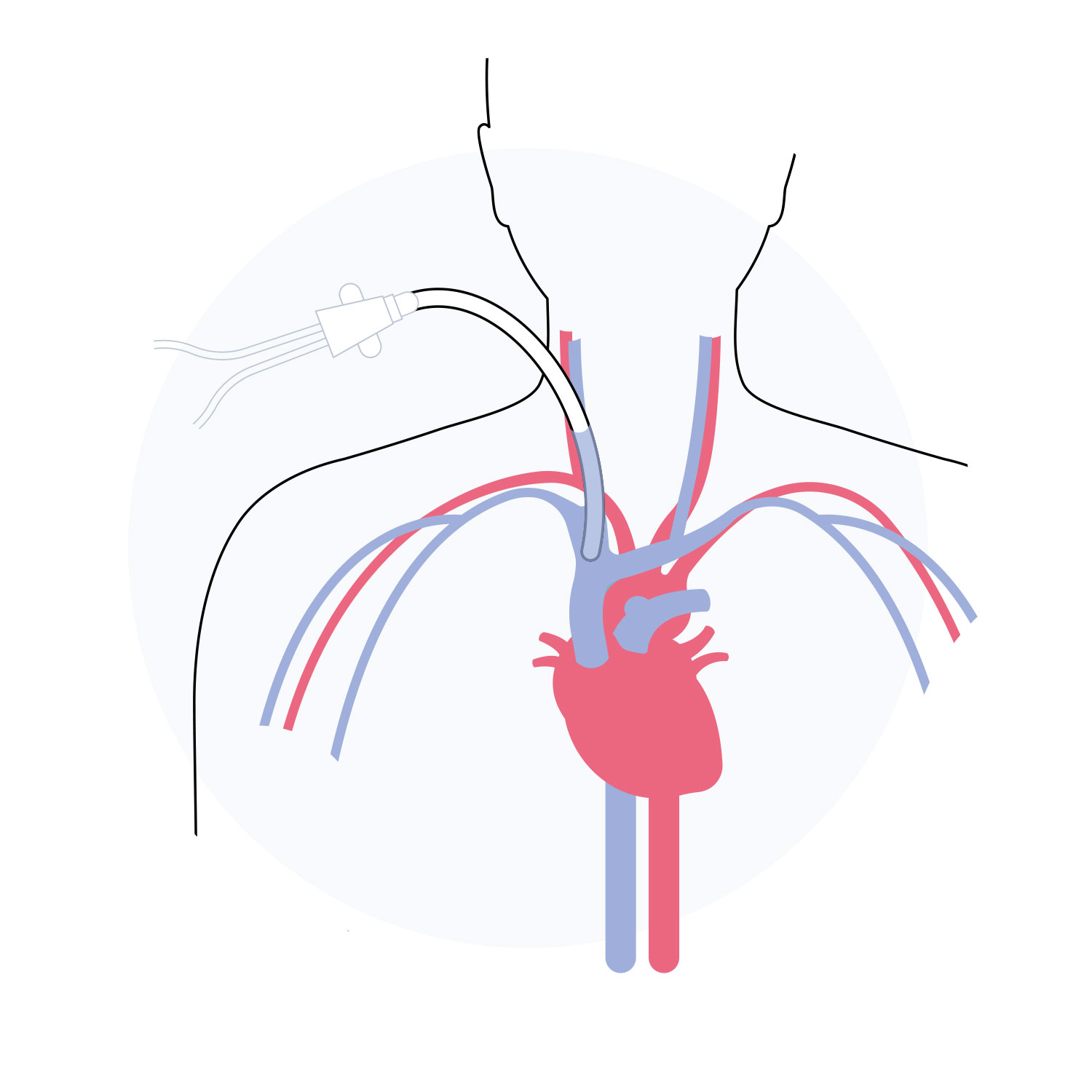
Around 30,000 patients per year, mostly children, suffer from congenital pulmonary valve heart disease globally.
1 in 5 pulmonary heart valves fail within a year
Current devices have poor performance and fail early due to narrowing of the heart valve because of stenosis, calcification and endocarditis leading to multiple early interventions.
The Solution
Pulmonary Heart Valve
Xplore-2 is a restorative pulmonary heart valve which turns into a living valve made of the patient’s own tissue.
Xeltis’ valve replacement has the potential to significantly reduce the reintervention rate associated with current devices, and the related burden to healthcare systems.
12-month clinical trial data showed no incidence of reoperation, reintervention or device-related death. Other trials are ongoing.
Visit xplore2md.com to learn more.
PIPELINE
Summary of our ongoing clinical programs
Xeltis’ clinical programs are seeking to address the significant challenges faced by vascular surgery patients, offering transformative implants with reduced rates of infection, rejection and need for reintervention.
Visit our Patients page for an overview of our ongoing clinical trials.
Patients



