Our Endogenous Tissue Restoration (ETR) platform
Xeltis’ implants enable a patient’s own natural healing system to restore a new blood vessel – a therapeutic process called Endogenous Tissue Restoration (ETR).
At the heart of Xeltis’ vascular restoration platform is Nobel prize-winning technology.
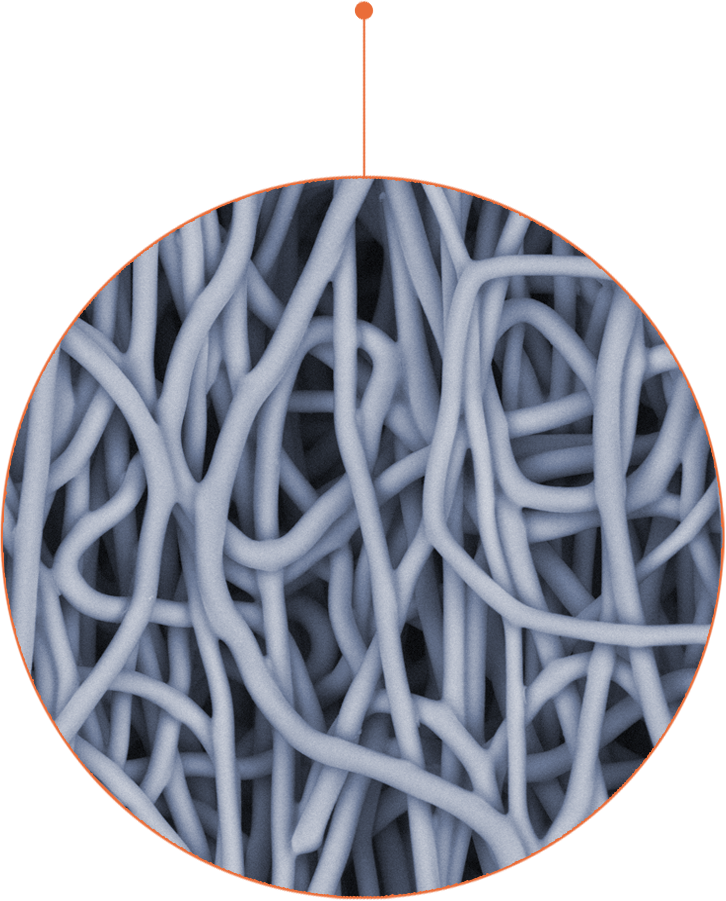
Using supramolecular chemistry, first developed from the laboratory of the renowned Prof. Jean-Marie Lehn, the novel polymer is electrospun into nanofibers to create implants which restore vascular function.

Xeltis’ groundbreaking platform is the most significant breakthrough in vascular replacement technology in over 60 years.

Over time, our implants are gradually replaced by the patients’ own living healthy tissue.

Xeltis’ implant technology is backed by strong research conducted over many years.
Key findings to date across preclinical and clinical research have been low infection rates, no occurrence of narrowing or stenosis and a consistent process of natural healing.
90
Implanted in >90 patients
20
>20 patients with 5 year follow up
50
>50 patients received aXess vascular access conduit
BENEFITS
Compared to existing treatments, Xeltis’ transformative vascular and valve implants offer:
Reduced reinterventions
Lower rates of infection and rejection
Scalability and ease of storage
Standard surgical techniques
Xeltis is developing medical devices to address key areas of unmet need in vascular replacement and break the cycle of complications and reinterventions.
Visit our Applications page to learn more.
Applications



